News Desk
Two hospitals in Uttar Pradesh have begun trials of the COVID-19 vaccine developed by the Bharat Biotech and in all 30 volunteers were administered the dose of the vaccine.
In Kanpur trial began at the Prakhar Hospital and in Gorakhpur it is Rana Hospital conducting trial with 10 volunteers.
The vaccine named Covaxin has been developed by Bharat Biotech and is being put under trial at 12 centres across the country. Two centres are in Uttar Pradesh. A 26-year-old from Kanpur became the first person to get the vaccine dose.
At the Rana hospital 10 volunteers were administered the dose of vaccine and have been asked to lead a normal life. They will be screened again after a gap of 15-days and if required early also. The hospital plans to conduct the trials on 100 healthy volunteers in two phases.
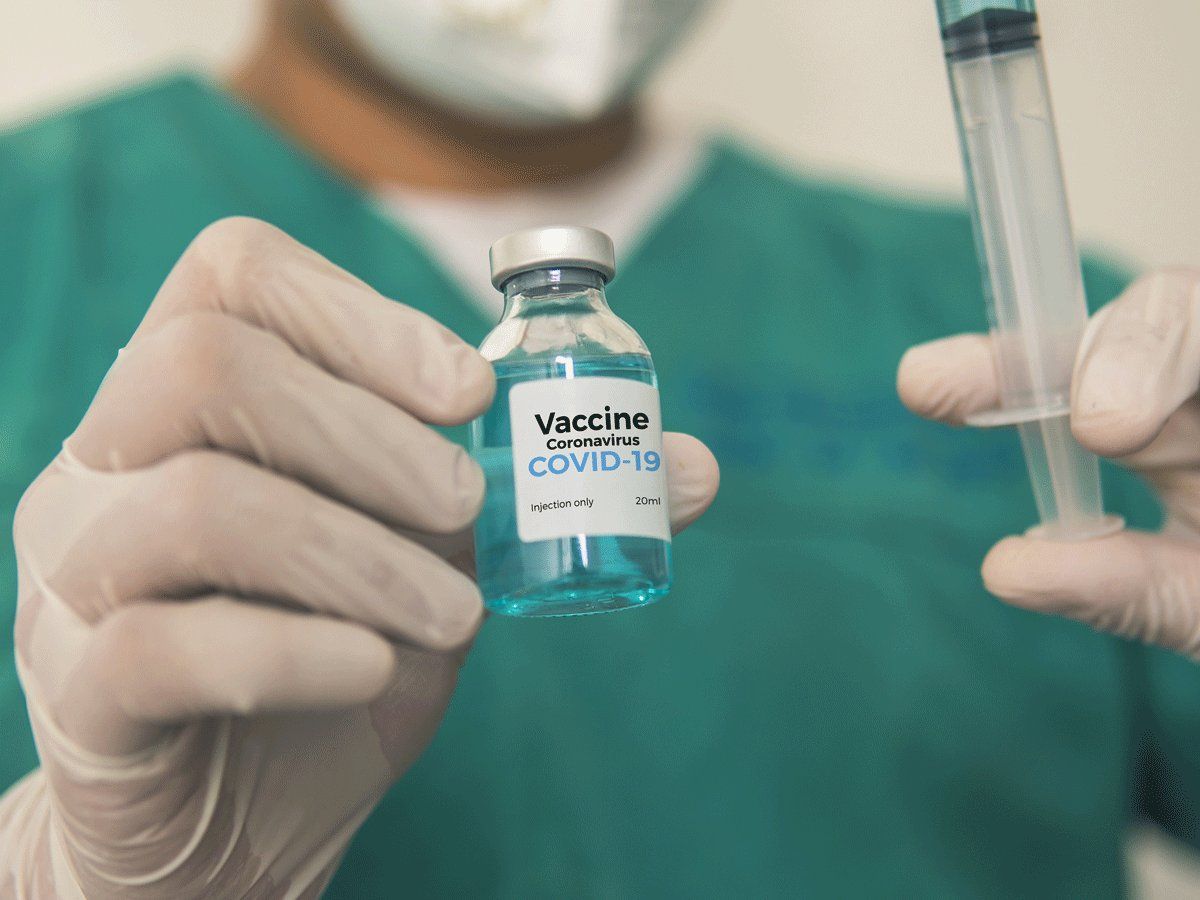
The volunteers are between 18 and 30 years of age. Before the trials the volunteers were screened for their fitness level and their samples were tested in Delhi lab. After due approval from the medical teams they were called for vaccination.
Also Read : Gehlot government can bring confidence motion
Also Read : First Covid-19 fatalities in Vietnam
COVAXINTM, is India’s indigenous COVID-19 vaccine by Bharat Biotech is developed in collaboration with the Indian Council of Medical Research (ICMR), National Institute of Virology (NIV). The indigenous, inactivated vaccine is developed and manufactured in Bharat Biotech’s BSL-3, high containment facility.
The vaccine received DCGI approval for Phase I & II Human Clinical Trials and the trials will commence across India from July, 2020.
Across the world scientists are working to bring out vaccine and France has signed a European agreement to pre-order up to 400 million Covid-19 vaccinations – should the current trials prove safe and successful – for possible public use before the end of 2020.
 Jubilee Post News & Views
Jubilee Post News & Views





